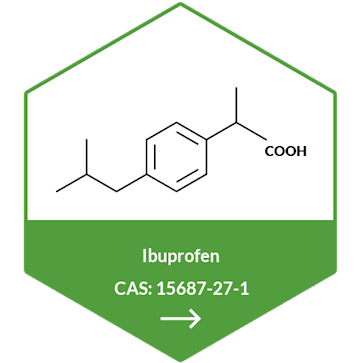
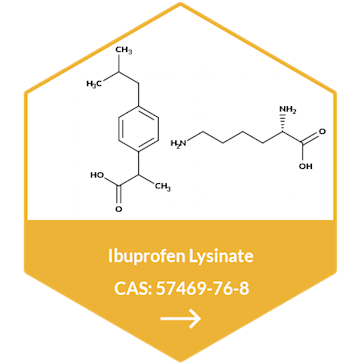
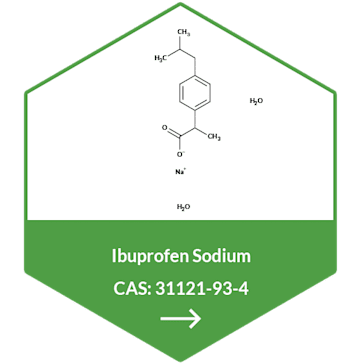
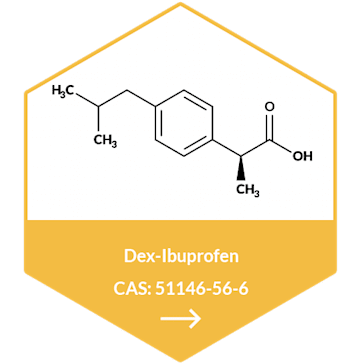
Metformin Hydrochloride API
Expertise in
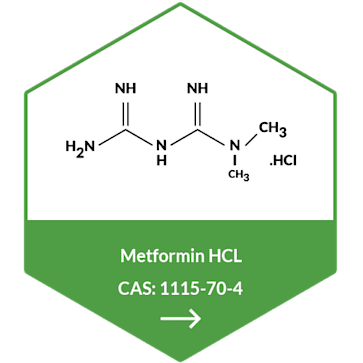
Metformin Hydrochloride
Dedicated manufacturing block to produce the API.
Highest quality standards that meet all Pharmacopeial and ICH requirements
deemed necessary for manufacturing.
Metformin synthesis process assures the NDMA content less than 1 ppb as
against a limit of 32 ppb.
Also, IOLCP has a great control on Dimethylamine which
is a precursor of NDMA formation during the formulation.
Contact Us
Contact Us
Expertise in
Metformin Hydrochloride
Dedicated manufacturing block to produce the API.
Highest quality standards that meet all Pharmacopeial and ICH requirements
deemed necessary for manufacturing.
Metformin synthesis process assures the NDMA content less than 1 ppb as
against a limit of 32 ppb.
Also, IOLCP has a great control on Dimethylamine which
is a precursor of NDMA formation during the formulation.
Mechanism of Action
Metformin hydrochloride modulates blood glucose levels by decreasing the hepatic glucose production (also called gluconeogenesis) and intestinal absorption of glucose and increasing insulin sensitivity by increasing peripheral glucose uptake and utilization.
API inhibits the mitochondrial complex I activity. After ingestion, the organic cation transporter-1 (OCT1) is responsible for the uptake of metformin into hepatocytes (liver cells).
It prevents the production of mitochondrial ATP which leads to increased cytoplasmic ADP: ATP and AMP:ATP ratios. These changes activate AMP-activated protein kinase (AMPK), an enzyme that plays an important role in the regulation of glucose metabolism.
Indication
The main therapeutic indications of API are:
Adults with type 2 diabetes mellitus can improve their glycemic control by using Metformin as an adjunct to diet and exercise.
Polycystic ovary syndrome is another condition for which metformin is prescribed.